Is Bcl3 Trigonal Planar
- the molecules are all aligned in one plane -. The molecular geometry of the BCl3 molecule is _____ and this molecule is _____-trigonal pyramidal polar-trigonal planar polar-trigonal bipyramidal polar-trigonal pyramidal nonpolar-trigonal planar nonpolar.

Hybridization Of Bcl3 Hybridization Of Boron In Bcl3
- 3 bonded pairs.

Is bcl3 trigonal planar. As per VSEPR notation this molecule takes AX3 notation. This analysis also predicts that the stretches should have A1 and E symmetry but since it has already been concluded that the molecule does not have C 3v symmetry this result will not be discussed further. Which of the following isare trigonal planar if any.
Therefore there exists no polarization of charges across the BCl3 molecule. Molecular shape of SiF6-2. Trigonal planar nonpolar tetrahedral unipolar tr.
The geometry of BCl3 is trigonal planar. The BCl 3 molecule is a flat trigonal planar molecule with 3 bonds and no lone pair of electrons on B. Its a 4 marker asking explain why a BF3 molecule has the shape you have drawn Please help.
- lone pairs repel more strongly than bonded pairs. Probably marks for saying. In addition BCl3 contains a h plane and three C2 axes see Figure 32.
What is the geometry of the molecular compound formed by the reaction of sulfur with hydrogen. BCl3 has 3 B-Cl single bonds and no lone pair around B hence 6 valence electrons around B. YOU MIGHT ALSO LIKE.
The three B-Cl bonds are evenly arranged in the same plane. It is composed of 1 boron and 3 chlorine atomThe bond angles are 120. Answer to What is the molecular geometry and the polarity of the BCl3 molecule.
B 15 Cl 30 it is likely that BCl 3. However if we look at its shape it is pyramidal and not planar unlike CH3 free radicalwhich is sp2 hybridised which signifies that it should have sp3 hybridisation. The molecule has three bond pair electrons which arrange themselves in a trigonal planar way to avoid the repulsion.
Identify the molecule that has polar bonds but is non-polar net dipole moment is 0 a. Determine the electron geometry eg and molecular geometry mg of BCl3 eg trigonal bipyramidal mg tetrahedral eg trigonal planar mg trigonal planar eg trigonal planar mg tetrahedral eg tetrahedral mg trigonal pyramidal. Phosphoryl chloride commonly called phosphorus oxychloride is a colourless liquid with the formula POCl3.
The three atoms bound to the central atom represent the vertices of a triangle. Wile in case of BCl3 actually the Boron atom has only 1s22s22p1 configuration and so if it combines with monovalent 3Cl -atoms its compound will have incomplete octet and so BCl3 will be electron deficient and hence its hybridization becomes sp2 with trigonal planer shape. What is molecular shape of BCl3.
In the Lewis structure for BCl3 the central atom Boron will only have six valence electrons. Bcl3 is a planar molecule whereas ncl3 is pyramidal because a nitrogen atom in smaller than boron atom b n cl bond is moe covalent than b cl bond c b cl bond is more polar than ncl bond d bcl3 has no lone pair of electrons where ncl3 has a lone pair of electrons. Note that Boron can have a full outershell with only six valence electrons.
This problem has been solved. BCl3 is trigonal planar use VSEPR and possesses all the above symmetry elements. - no lone pairs.
Boron trichloride BCl3 is characterized by trigonal planar molecular shape. - repel each other equally at 120 degrees. BCl3 is a trigonal planar molecule like the other boron trihalides and has a bond length of 175pm.
It is a flat molecule with all three bond angles 120 degree C. Linear angular trigonal planar tetrahedral please explain the correct answer. What is POCl3 called.
The BCl3 molecule is considered to be non-polar because the charge distribution across the molecule is uniform as the shape of the molecule is symmetric ie. This is not in agreement with the experimental data so BCl3 does not have trigonal pyramidal geometry. For more information you must also go through an article on the polarity of BCl3.
The Correct Answer is.

Why Is Bcl3 Trigonal Planar In Shape Whereas Anhydrous Alcl3 Is Tetrahedral In Shape Chemistry Chemical Bonding And Molecular Structure 12837253 Meritnation Com
What Is The Structure Of Bcl3 Quora

Is Bcl3 Polar Or Nonpolar Techiescientist
Bcl3 Lewis Structure Molecular Geometry Hybridization And Shape
What Is The Structure Of Bcl3 Quora
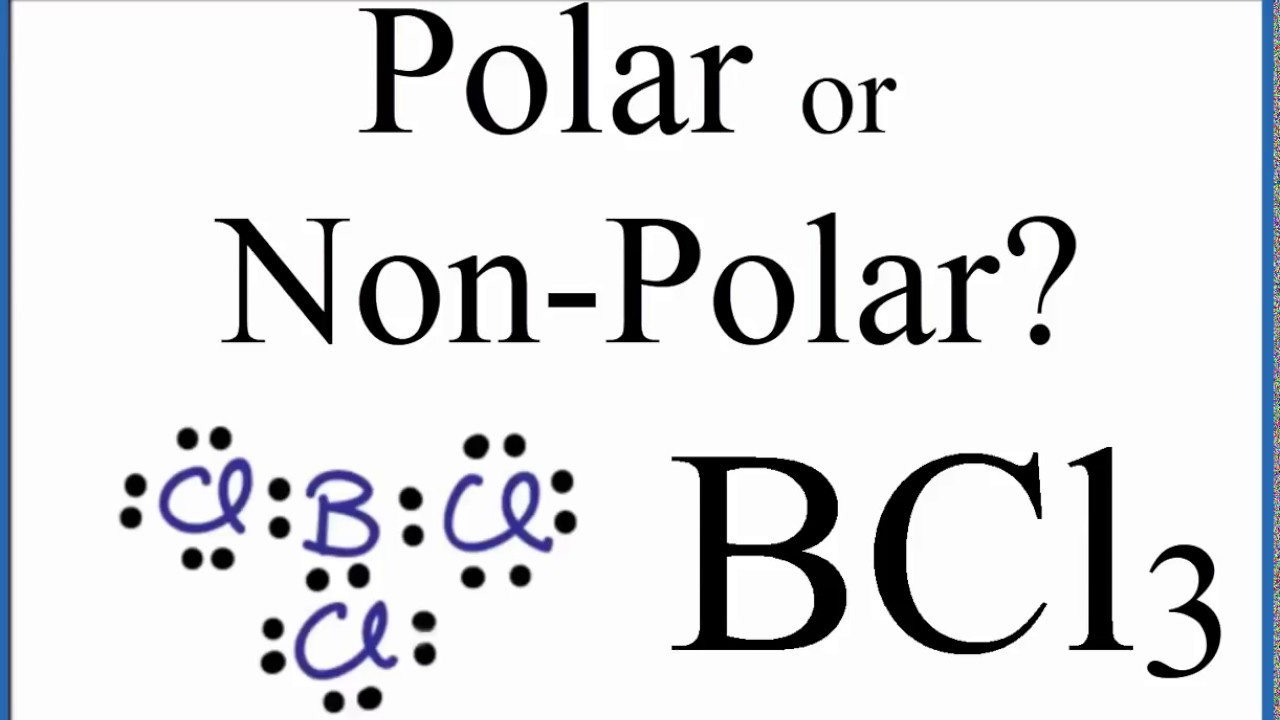
Is Bcl3 Polar Or Non Polar Boron Trichloride Youtube
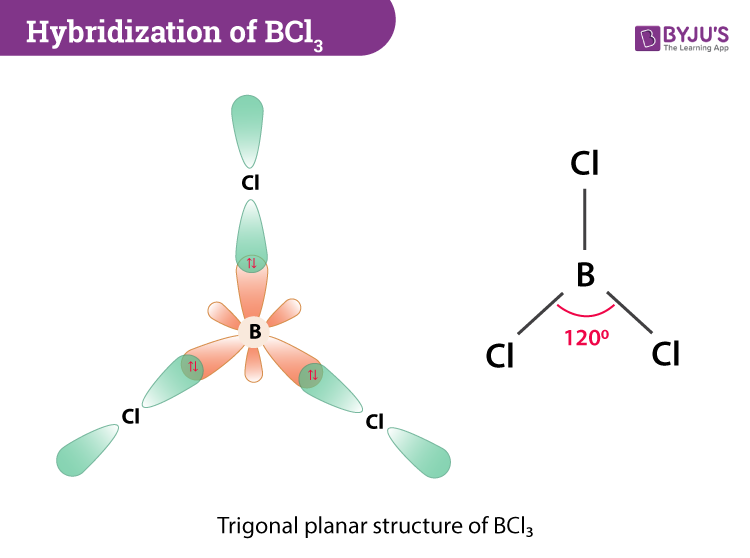
Hybridization Of Bcl3 Hybridization Of Boron In Bcl3

Using Vsepr To Determine Molecular Shape Bcl3 Intermolecular Forces Meristem Youtube
0 Response to "Is Bcl3 Trigonal Planar"
Post a Comment