Predict The Shape Of Bcl3
What is the molecular shape of ClF4-Square planar. What is the molecular shape of SiF6-2.

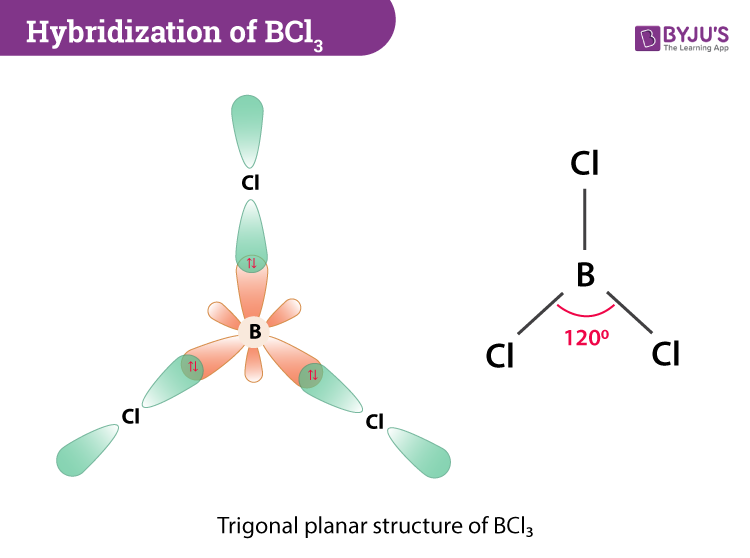
Hybridization Of Bcl3 Hybridization Of Boron In Bcl3
Thus H 33 6 3 Sp2 hybridization Therefore its clear from the formula as well that BCl3 has Sp2 hybridization.
Predict the shape of bcl3. In SiCl 4 the central atom has no lone pair and there are four bond pairs. A linear B trigonal planar C bent D tetrahedral E trigonal pyramidal. Ii The shape of C H 4 is t e t r a h e d r a l carbon is s p 3 hybridized.
Each chlorine atom uses its half filled p-orbital for the -bond formation. XNumber of surrounding atoms. Each group around the central atom is designated as a bonding pair BP or lone nonbonding pair LP.
It will be 1s 2 2s 2 2p 1. BeCl2 BCl3 SiCl4 AsF5 H2S and PH3. What is the molecular shape of BCl3 as predicted by the VSEPR theory.
In this case one electron from the 2s is moved to the 2p level. Use lewis structure guidelines to draw the lewis structure of BCl 3. Hence it has a linear shape.
I In B C l 3 Boron is s p 2 hybridized and thus the shape of B C l 3 is t r i g o n a l p l a n a r. When lone pair electrons are present the parent structure are. Which series correctly identifies the hybridization of the central atom in.
Boron forms three sp-p bonds with three chlorine atoms by using its half filled sp 2 hybrid orbitals. What are the rules of valence shell electron pair repulsion VSEPR. CCl4 H2S CO2 BCl3 Cl2 Why is molecular geometry important.
To form bonds with chlorine boron will require three unpaired electrons. C and A would be 0 as BCl3 is a neutral compound. Click hereto get an answer to your question Determine the shape of the following molecules using the VSEPR model.
Is PCl3 polar or nonpolar. Hence the shape of SiCl 4 is tetrahedral being the AB 4 type molecule. A step-by-step explanation of how to draw the BCl3 Lewis Dot Structure Boron trichlorideFor the BCl3 structure use the periodic table to find the total nu.
Hence it is trigonal planar. The polarity of any given molecule depends on its molecular geometry net dipole moment in the molecule and lone pairs in the molecule. In the VSEPR model the molecule or polyatomic ion is given an AX m E n designation where A is the central atom X is a bonded atom E is a nonbonding valence electron group usually a lone pair of electrons and m and n are integers.
Hence it is of the type AB 3. What is the molecular shape of SCl_2. What does molecular shape of NOCl.
AX 3 has trigonal planar shape. For the above molecule VSEPR notation will be AX 3 E 0. ANumber of central atoms.
BCl3 is SP2 hybridisation and hence its structure is trigonal planner. Which of the following has a T-shaped structure. Predict the shapes of the following molecules on the basis of hybridisation BCl3CH4CO2NH3.
Hence it is trigonal planar. Hence the shape of SiCl4 is. A BF3 molecule is trigonal planar because its three fluorine atoms around a central boron atom and that boron has no lone pairs on it.
The hybridization of BCl 3 now occurs where one 2s and two. BCl3 takes the shape of trigonal planar. Hence it has a linear shape.
According to VSEPR theory the molecular geometry of boron trichloride is trigonal planar with a bond angle of 120 degrees. Hence it is of the type EX3. How can I draw the Lewis dot structure for BeF2.
What is the shape of ClO3F as predicted by VSEPR theory. Predict the electron group arrangement around iodine in IF2-. The central atom has no lone pair and there are four bond pairs.
From Gargi College University Of Delhi 2021 Answered 2 years ago. Thus the shape of BCl 3 is trigonal planar with bond angles equal to 120 o. Which of the following has square-planar molecular geometry.
What is molecular shape of BCl3. Now the electron configuration will be in an excited state and will be represented as 1s 2 2s 2 2px 1 2py 1. Lone pair electrons are also taken into account.
The central atom has no lone pair and there are three bond pairs. We will look at the ground state of borons electron configuration. The central atom has no lone pair and there are three bond pairs.
Phosphorus Trichloride has a trigonal pyramidal shape as the electrons are arranged in a tetrahedral geometry. E Number of lone pairs on central atom. Use VSEPR table to find the shape.
Apply VSEPR notation A X E. This gives it AX3 V. See all questions in Molecular Geometry.
What Is The Molecular Geometry Of Bcl3 Quora

Using Vsepr To Determine Molecular Shape Bcl3 Intermolecular Forces Meristem Youtube

Bcl3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

Bcl3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

Bcl3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

Bcl3 Lewis Structure Molecular Geometry And Hybridization Techiescientist
Hybridization Of Bcl3 Hybridization Of Boron In Bcl3
What Are The Hybridisation And Shape Of Bcl3 Quora
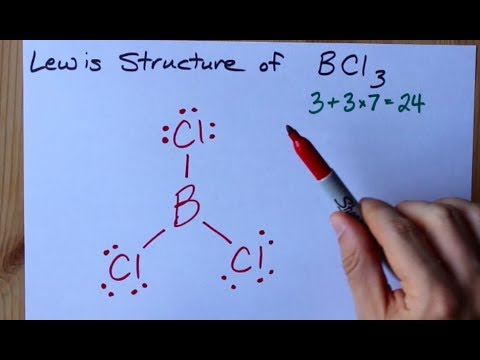
How To Draw The Lewis Structure Of Bcl3 Boron Trichloride Youtube
0 Response to "Predict The Shape Of Bcl3"
Post a Comment